How do clouds look like and what are the made of on exoplanets and brown dwarfs? Are they as interesting as here on Earth? We certainly think yes! Graham Lee and his collaborators’ paper has just been published on the topic of modelling the formation of condensation seeds. This work involved two summer students under the supervision of Dr Christiane Helling. Here comes a brief summery of their work.
“Rain may fall, and wind may blow
And many miles be still to go
But under a tall tree will I lie
And let the clouds go sailing by”
– J.R.R. Tolkien
Clouds are an everyday experience for most humankind here on Earth. We admire the sheer span of the objects and the sometimes recognisable and/or funny shapes they form in the sky. When they turn dark we know rain is on the way and take shelter. Yet we also know that our main source of fresh water comes from the traveling of clouds many miles from ocean to land. However, despite centuries of investigation by scientists, the exact physics of clouds remains elusive and not fully understood. In recent years, astronomers have begun to notice the effect of clouds when observing exoplanets and low-mass stars such as brown dwarfs. Just like on Earth, these clouds are very important to the eco-system and environment of the objects. Unlike Earth, these clouds are not like our everyday water clouds but rather are composed of a multitude of a multitude of solid materials like silicates and iron. Clouds play a key role in the energy budget of a planet, either reflecting heat away or trapping heat inside the planet’s atmosphere. It has, therefore, become more and more important to accurately describe and observe in detail the clouds found on these planets. This has implications on the habitability of such planets.
Modelling hot Jupiter exo-clouds
Every cloud particle has a beginning, and in hot Jupiter atmospheres a potential cloud particle starts off as a single gas molecule called a monomer. The main monomer of interest to our research is TiO2 (Helling & Fomins 2014). At the upper edges hot Jupiter atmospheres conditions can reach 500 K where the formation of small clusters made of TiO2 molecules starts.
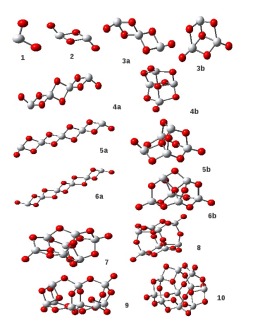
TiO2 molecule geometries. Molecules labelled ‘a’ are the old polymer like chain geometries from Jeong et al. 2000. Those labelled ‘b’ or unlabelled are the newer geometries from Calatayud et al. 2008 and Syzgantseva et al. 2010.
A TiO2 gas molecule then chemically bonds with another TiO2 molecule creating a 2 monomer gas particle (TiO2)2. Further TiO2 monomers can then bond with this 2 monomer gas particle, step by step increasing the size of this gas particle of these molecules which are called ‘clusters’. When the chemical chain reaches a critical monomer number N, it is large enough to be called a cloud “seed particle” and considered to be in the solid rather than gaseous state. This rate of growing gas molecules until they are solid is called ‘nucleation’. The process is key to the formation of clouds, as without the nucleation of a seed molecule, no further material can condense onto its solid surface.
Once the seed particle is formed other materials condense onto the grain surface causing the cloud particle to grow in size. As the cloud particle grows it falls due to gravity to deeper, hotter and denser regions of the atmosphere. This accelerates the growth process as more material is available to condense onto the surface. Particles can grow to micro meter sizes. Due to this condensation and nucleation the local gas is depleted of elements that constitute the compositional make up of the cloud particle. Once the temperature becomes too high for thermal stability of the cloud particle materials, they evaporate from the surface until all solid material returns to the gas phase. Convection from deeper atmospheric layers provides element replenishment to upper, cooler layers allowing the cloud formation process to reach a stationary state.
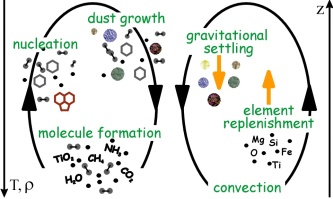
Figure 1. Nucleation (seed formation), dust growth (and evaporation), gravitational settling (rain-out) and element replenishment are processes involved into the formation of a cloud. The inner part of an atmosphere is typically warmer than the outer part in a brown dwarf, and no cloud particles can form. Atmospheres of brown dwarfs and giant gas planets are convective (”boiling”) which provides the mechanisms for element replenishment. Original figure in Woitke & Helling (2004).
The typical observable signatures of cloud in these hot Jupiters are the characteristic Rayleigh scattering of light from the cloud in its atmosphere (e.g. Wasp-31b; Sing et al. 2014, HD 189733b; Pont et al. 2013). Other signatures are a flat, featureless spectrum, which indicates thick cloud cover, obscuring hot gas underneath. This flat signature might also be attributed to the element depletion effects of cloud formation. If an element is depleted from the atmosphere of a hot Jupiter it is harder to detect.
In Lee et al. 2014, we looked again at our cloud formation model to see how our predicted cloud structures changed with the addition of new chemical data for TiO2 and SiO molecules. These two molecules are considered the most likely to nucleate and form seed particles in hot Jupiter atmospheres. Our aim was to find out if this new data had an impact on our previous assumptions that TiO2 was the main nucleation species in exoplanet atmospheres. Using new TiO2 geometrical cluster data from Calatayud et al. 2008 and Syzgantseva et al. 2010, we performed computer quantum chemical calculations called density functional theory on these molecules.
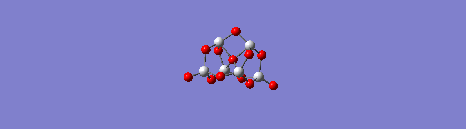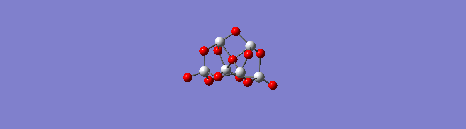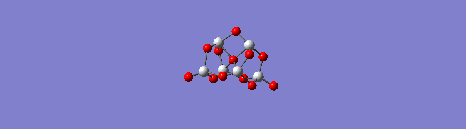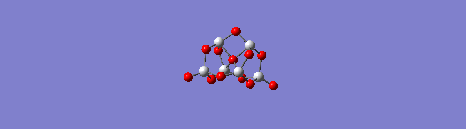
Movie showing one of the vibrational modes of the (TiO2)6 structure resulting from the quantum mechanical calculations.
We also switched our primary nucleation seed to SiO using new experimental vapour data (Wetzel et al. 2013). Our cloud model relies on accurate values on how much energy is required to nucleate these particles and to estimate their macroscopic properties. We found that the new geometries of TiO2 molecules enhanced the rate of nucleation compared to the old values (from Jeong et al. 2000). Furthermore, SiO proved to be the most efficient nucleation species several times that of TiO2.
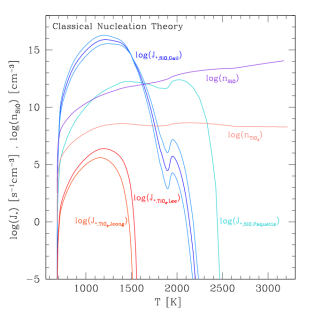
Number densities (cm−3) and nucleation rates J_(star) (s−1cm−3) for both TiO2 and SiO. Red lines indicate TiO2 nucleation using various data. Blue lines indicate SiO nucleation. SiO nucleates at a higher rate than TiO2 in isolation. This does not take into account feedback effects from element depletion.
This was primarily due to the greater abundance of SiO compared to TiO2 in the gas phase. However, after the formation of the seed particle is complete, materials such as SiO and SiO2 can condense onto the grain surface. This depletes the local gas of Si elements which in turn reduces the rate of SiO nucleation, since less material is available to nucleate. TiO2 on the other hand, condenses onto grain surfaces at much slower rates, leaving enough material to nucleate. This makes TiO2 nucleation overall more efficient and the primary seed particle of these clouds.
In conclusion, clouds play a vital role in the ecosystems and environment of whatever objects they are found on. Atmospheric cloud modelling requires careful treatment of the seed particles, from our Solar System planets to exoplanets. Future molecular data may change the specific qualities of these seed particles and we hope to continue to refine and improve this important aspect of cloud modelling.
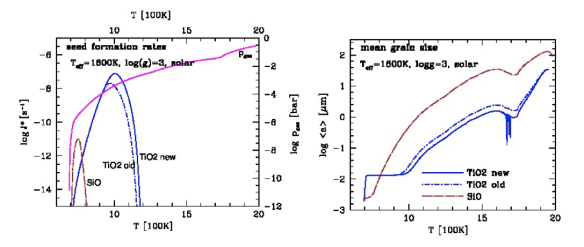
file_ameanSiOTiO2individual_16003 TiO2- and SiO-nucleation rates (top) calculated as part of the cloud formation model and their effect on the number density of cloud particles (middle) and the mean grain size (bottom). The calculations include nucleation, growth/evaporation, element conservation, gravitational settling and convective replenishment. TiO2 nucleation produces more seed particles and at a higher rate than SiO in our atmospheric models due to the depletion of SiO in the gas phase from grain growth.
Additional information:
Solar system planets
It is not a surprise when we learn as a child that clouds on Earth are made of water ice particles. After all, water is a major constituent of the Earths geological make-up and Earth’s temperature conditions are such that water can exist in solid, liquid and gaseous form. However, water vapour makes up a tiny fraction of the Earth’s atmosphere (less than 0.1 %) compared to the main gases; Oxygen (21 %) and Nitrogen (78 %). Each cloud particle on Earth contains a micrometer sized seed particle of sand or ash at its core, kicked upwards by winds or volcanic eruptions. It is when water vapour reaches freezing point in the troposphere that it condenses onto the surface of these seed particles, forming our well known water clouds. Similarly, for other planets their types of cloud depend on the local gaseous content available, the creation of a suitable trace molecule to condense/freeze and the local thermodynamic conditions these molecules find themselves in.
For Venus, the clouds that cover its atmosphere are so thick that its rocky surface is hidden from view. The clouds of Venus also make it the hottest planet in the Solar System as they help trap heat deep within the atmosphere. The atmosphere is mostly composed of carbon-dioxide (97 %) and Nitrogen (3 %) with trace elements making up less than 1 per cent of the composition. It is known that the main constituent of clouds on Venus are sulphuric acid (H2SO4) droplets formed from ultra-violet chemical reactions by radiation from the Sun. Interestingly, when these droplets fall due to gravity they do not reach the surface of Venus since at a height of ~30km from the surface these droplets evaporate because of high temperatures. It is thought that the chemicals and seed particles for this sulphuric acid rain come from the eruption of surface volcanoes.
Jupiter, Saturn, Uranus and Neptune all have similar atmospheric compositions dominated by Hydrogen and Helium but all have different cloud structures and compositions. The surface temperatures of Jupiter, Saturn, Uranus and Neptune are approximately 165, 134, 76 and 72 K respectively. These temperatures are much cooler than Earth (approx. 288 K) so we expect a vastly different cloud composition and structure in these objects and also differences between larger and smaller gas planets . In Jupiter and Saturn there are several molecules that can condense to form clouds in these atmospheres including, NH3, NH4SH and H2O. On Uranus and Neptune we also expect CH4 and H2S clouds to form in addition to the clouds found on Jupiter and Saturn. It is thought that the water clouds in these objects can produce lightning similar to Earth’s but at a much higher intensity, releasing as much as 1000 times the average energy of a lighting strike on Earth.
For more details check out the original paper on ADS:
Lee G., Helling Ch., Giles H., Bromley S.T., A&A 575, A11
The LEAP Group can be found here:
http://leap2010.wp.st-andrews.ac.uk/
And finally, don’t forget to like us on Facebook:
